Certificates
-
Standard: ISO 13485: 2016Number: 381210214R0SIssue Date: 2021-12-28Expiry Date: 2024-11-27
-
Standard: ISO 9001: 2015Number: FM 564641Issue Date: 2010-11-10Expiry Date: 2025-07-29
-
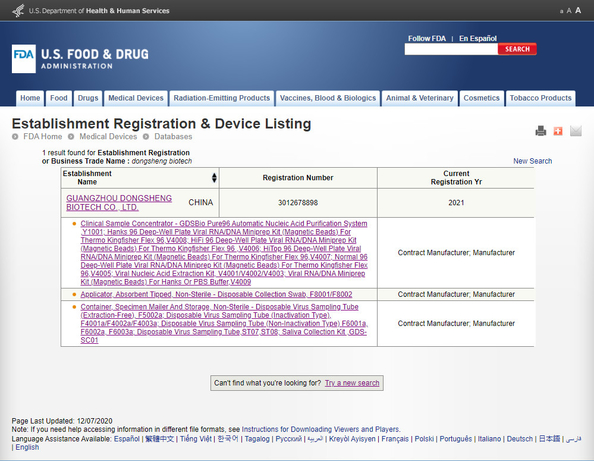
Standard: FDA registrationNumber: 3012678898Issue Date: 2021-05-12Expiry Date: 2025-12-31
-
Standard: Business licenseNumber: 91440106771176231LIssue Date: 2022-01-21Expiry Date: 2026-01-21
-
Standard: AUDITED SUPPLIERNumber: QIP-ASI211880Issue Date: 2021-08-30Expiry Date: 2025-08-30
-
Standard: CE certificate - sampling tubeNumber:Issue Date:Expiry Date:
-
Standard: CE certificate - viral nucleic acid extraction kitNumber:Issue Date:Expiry Date:
-
Standard: CE certificate - COVID-19 nucleic acid detection kitNumber:Issue Date:Expiry Date:
-
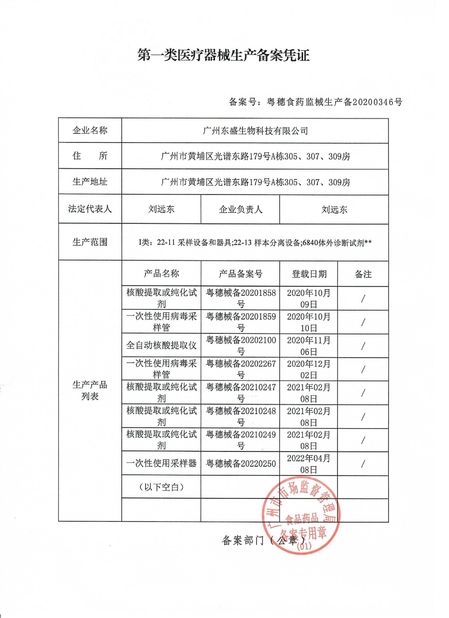
Standard: CFDA record - productionNumber:Issue Date:Expiry Date:
-
Standard: CFDA record - viral nucleic acid extraction kitNumber:Issue Date:Expiry Date:
-
Standard: CFDA record - sampling tubeNumber:Issue Date:Expiry Date:
QC Profile
GDSBio has established a quality management system and obtained ISO 9001 and ISO 13485 certifications. The system contains QC documents to control the quality inspection process of products. The basic process is as follows:
![]()
Every product has passed strict quality inspection.
![]()